What Are the Core Electrons of Strontium
Electrons due to their smaller mass and thus larger space-filling properties as matter waves determine the size of atoms and molecules that possess any electrons at all. This is incorrect because metals still consist of atoms but the outer electrons are delocalised and are free to move through the structure.

Webelements Periodic Table Strontium Properties Of Free Atoms
It has a silver appearance but then turns yellow with the formation of oxide.

. In a similar way graphite a non-metal also has delocalised electrons. However you dont see. Strontium is a group 2 element that does not occur as a free element due to its extreme reactivity with oxygen and water.
It occurs naturally only in compounds with other elements such as strontianite. Metallic bonding is often described as the attraction between positive metal ions and delocalised electrons. Thus anions negatively charged ions are larger than the parent molecule or atom as the excess electrons repel each other and add to the physical size of the ion because its size is determined by its.
For each atom the subshells are given first in concise form then with all subshells written out followed by the number of electrons per shell. It is softer than calcium and decomposes water more vigorously. This page shows the electron configurations of the neutral gaseous atoms in their ground states.

Sr Strontium Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
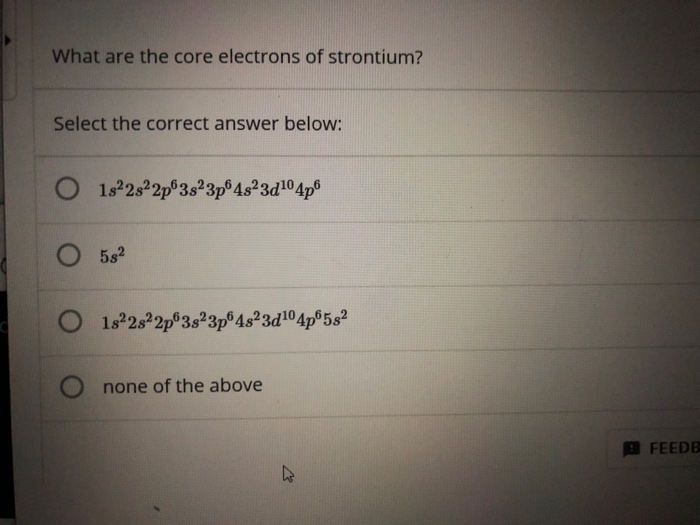
Solved What Are The Core Electrons Of Strontium Select The Chegg Com

Comments
Post a Comment